Mineral Monday + Tectonic Tuesday. Blue quartz is uncommon and is usually
colored by inclusions of unusual minerals like crocidolite, tourmaline, or
dumortierite. The purplish-blue quartz here, from north of Llano, Texas, is
colored by inclusions of ilmenite (iron-titanium oxide). This rock is called
llanite for its occurrence in the Llano Uplift of central Texas, and although
similar rocks are found in other parts of the world, the variety name llanite
really only applies to this location. On a sunny day, the blue quartz in the
rocks has an opalescent sheen that sometimes seems to “wink” at you from the outcrop.
More generally, the rock is a rhyolite porphyry – rhyolite meaning
pretty high in silica (a granite-like composition) and formed at or near the
surface of the earth, and porphyry meaning it has two grain sizes – a fine
matrix, with larger crystals of quartz (and microcline feldspar) suspended in
that matrix. This implies that there were two periods of cooling, one at deeper
depths where it took the larger crystals a longer time to cool (and grow),
followed by a later, quicker period of cooling, so the matrix crystallized so
fast the grains are very small, but the larger, older grains are still there
within the matrix.
All that cooling happened about 1,093,000,000 years ago
(almost 1.1 billion) during a time called the Grenville Orogeny (orogeny means
mountain-building) when what is now central Texas was amalgamated to the main
part of the North American continent. The llanite was probably an aspect of the
intrusions of the Town Mountain Granite, which has similar age but crystallized
at greater depth. It’s part of a long belt that extends with some discontinuity
to central Tennessee, through Kentucky and Ohio, then northeast across Ontario,
Quebec, and into Labrador. Rocks now in southern Scandinavia were part of the
Grenville mountain belt, the result of a collision between continental masses
that was assembling the supercontinent Rodinia over a long period of time, from
about 1,250 million years ago to 980 million years ago.
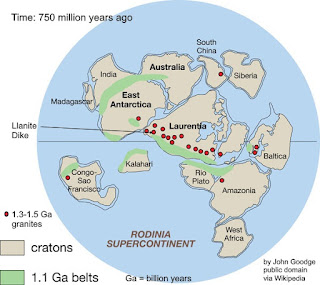
The llanite, in the form of a narrow dike, intruded older
rocks toward the end of the Grenville Orogeny. The mountain belt continued into
Mexico, and rocks of similar age are found in Australia and Antarctica as well
as South America today. Exactly how those rocks fit into the big picture is
still debated, but one version of the assembled continent of Rodinia is in the
comments.
At left, one reconstruction (others exist) of Rodinia about 750 million
years ago, just before it began to break up. “Rodinia” is from Russian for “motherand,”
or “to give birth,” alluding to this continent’s early place in the rifting-collision
cycles (called Wilson Cycles with respect to ocean basins, for J. Tuzo Wilson)
that have followed. Even so, Rodinia was probably preceded by at least one
earlier supercontinent, named Columbia – but that’s debated.
The llanite dike intrudes older Precambrian metamorphic rocks
called the Valley Spring Gneiss, which have a lot of magnetite in them. The
Valley Spring Gneiss is dated to about 1,375,000,000 years ago or older.

The phenocrysts (“showing” or “visible” crystals) of blue
quartz in the llanite are supposed to be “beta quartz,” a high-temperature,
higher symmetry form of quartz that can crystallize only above 573ºC – in fact,
it cannot even exist at surface conditions and pressures, so all “beta quartz”
is actually a pseudomorph (“false form”) of regular quartz that has formed as
the original beta quartz cooled. This little crystal (not from Llano) is probably
one such pseudomorph. (Technically, since I knew you wanted even more jargon,
it’s a paramorph rather than a pseudomorph, because while the crystal structure
has changed, the chemistry has not.) The essentially perfect hexagonal symmetry
of this crystal marks it as a beta-quartz shape, versus the lower (trigonal)
uneven symmetry typically displayed by normal quartz. It’s possible for normal
trigonal quartz to have equally developed faces that appear fully hexagonal,
but it’s unusual.